X-ray micro-CT Analysis of Lyophilized Cake Structures
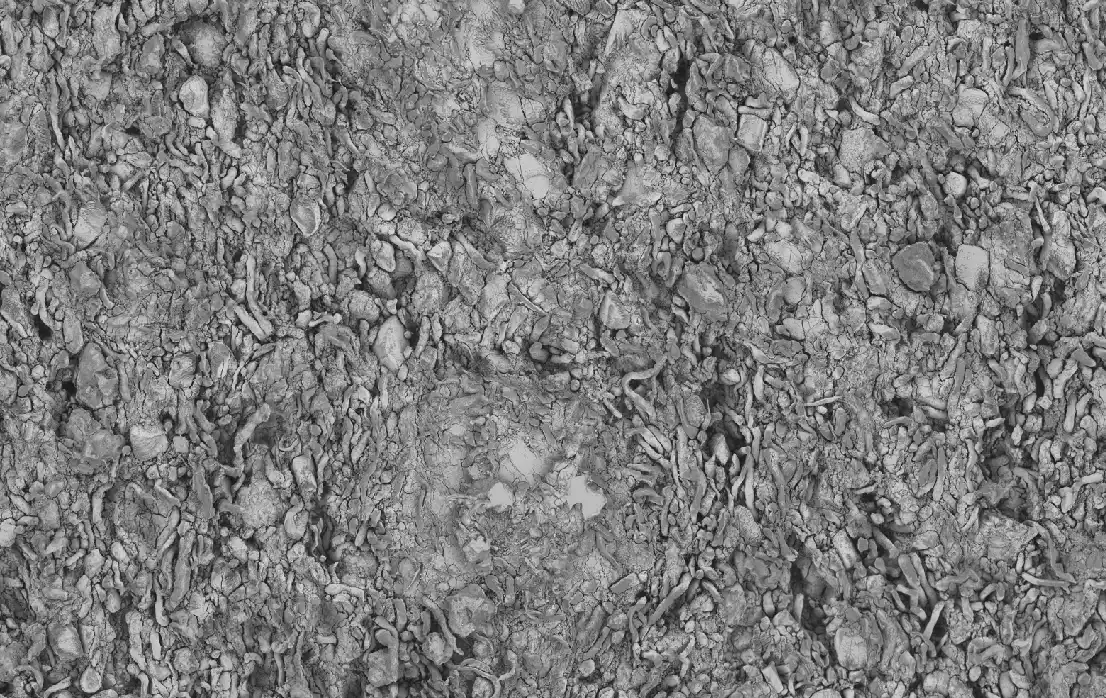
Non-Invasive Quantitative Characterization of Lyophilized Drug Product Using Three-Dimensional X-Ray Microscopy Analytics
Lyophilization is a freeze-drying process that removes water from a drug product via sublimation. It is a common and important processing method for pharmaceutical products that are not stable in liquid or frozen state, or when cold supply chain strategies are not applicable. Thorough characterization of drug product quality, such as cake moisture content, API purity, and cake appearance has to be conducted before market release. Cake appearance can be a simple way to elucidate critical product defects, but visual inspection may not detect impactful changes in the cake microstructure. An innovative image-based analytical method uses 1) high-resolution, non-invasive three-dimensional imaging with X-Ray Microscopy, 2) artificial intelligence-based image processing, and 3) image-based physical property modeling with direct numerical simulations. This new method can not only characterize quantitatively the microstructures of the lyophilized drug samples, but also presents the potential to correlate the microstructures with physical properties to optimize parameters in drug formulation, cycle development, process scale-up, stability control, and administration.

Shawn Zhang, John Goldman, Xiaodong Chen, Jasmine Rowe, Sam Lin, Liping Zhou
With BMS and Moderna
https://drug-dev.com/image-based-characterization-non-invasive-quantitative-characterization-of-lyophilized-drug-product-using-three-dimensional-x-ray-microscopy-analytics/
Additional Publications
Transform Your Program with Microstructure Science
Get started with a drug product digital twin.















