DigiM I2S Software
I2S, or Image to Simulation, is a cloud based image processing platform designed to manage the complete lifecycle of your image data.

Data Management
Utilizing a cloud based data management library, I2S allows its user to access image data anytime, anywhere, from any device. I2S data management includes FDA CFR 21 Part 11 compliance, by storing and maintaining these records I2S will automatically and track all data created, modified, and retrieved from within the platform for the data's entire lifecycle.

Image Segmentation
Image segmentation plays an important role in any image analysis workflow. I2S utilizes machine learning and deep learning based techniques to support the digital transformation of your drug products. I2S offers the tools to handle any type of image analysis problem.
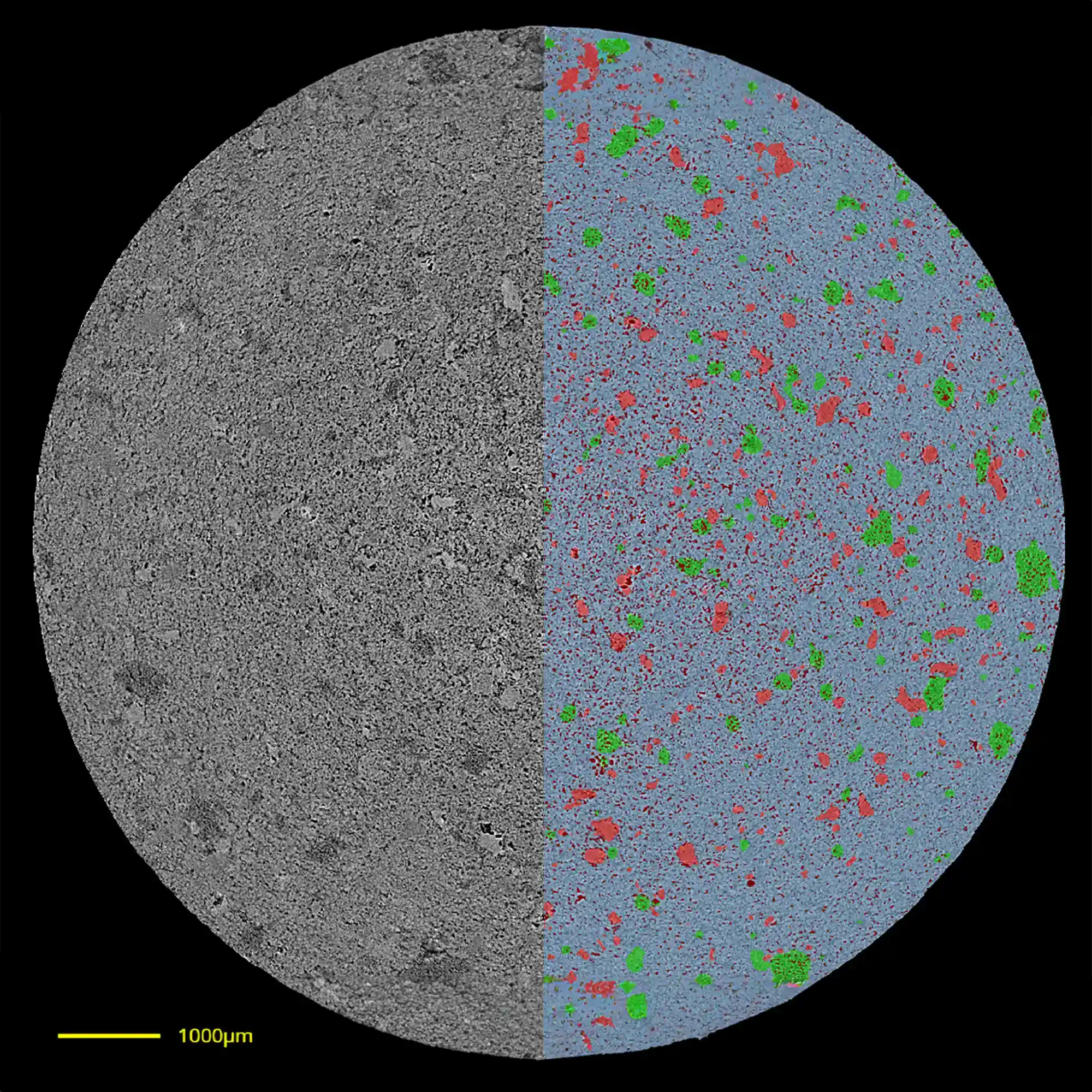
Microstructure Analysis
Once segmentation has been performed to create a digital twin of a drug product, an assortment of quality attributes can be computed from the twin. Morphological characterizations can be performed to determine metrics such as volume, particle size distribution, and spatial distributions, and many more.

Image-Based Simulations
I2S, features a suite of physics based simulations allowing a user to compute mass transport properties of there product directly from their imagery. digiM has developed a voxel based simulation method, in which each pixel of the scene is used as a computational cells to assist in these computations. This voxel based approach allows I2S users to compute highly accurate mass transport properties much faster than traditional fluid dynamics simulation techniques. I2S also contains a patented diffusion simulation allowing users to predict the drug release of the digital twins of their products.

Transform Your Program with Microstructure Science
Get started with a drug product digital twin.















