Brand vs Generic IUS systems
Microstructure Bioequivalence Assessment for Brand vs Generic Levonorgestrel IUS

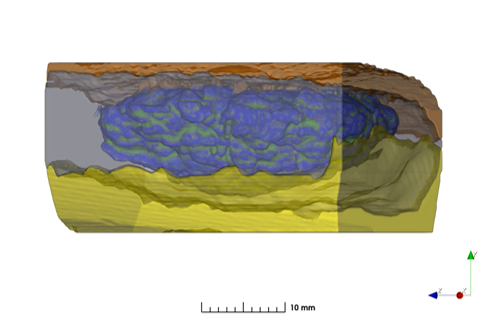
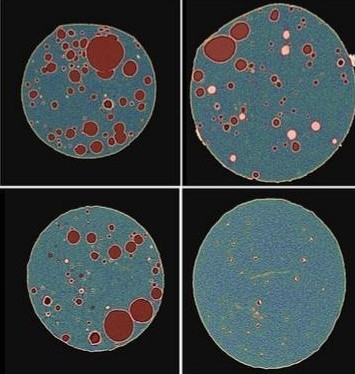
In this study, an in-house levonorgestrel (LNG) intrauterine system (IUS) was prepared following the same formulation (i.e., qualitatively (Q1) and quantitatively (Q2) the same) and compared side by side with the commercially available Mirena IUS device (the reference listed drug (RLD)) under stressed in vitro release condition and via micro-imaging technologies. Focused Ion Beam – Scanning Electron Microscope (FIB-SEM) was used to analyze two IUS samples (in-house vs. Mirena) and for each IUS sample, one device prior to release and one post completed release were analyzed with either 2D or 3D FIB-SEM.

The collected images were then analyzed by artificial intelligence-based image analytics to evaluate Q3 microstructure bioequivalence. The active pharmaceutical ingredient (API) particle size distribution, spatial uniformity, volume fraction of each material phase and permeability were assessed based on the high-resolution FIB-SEM images. Energy dispersive X-ray spectroscopy (EDS) confirmed the material phases in these samples.
Additional Case Studies
Our Expertise
In Numbers
Programs Supported
Formulations Digitized
Pharmaceutical Partners

Transform Your Program with Microstructure Science
Get started with a drug product digital twin.